What Element Is Used to Create the Blue Color in Fireworks

On Wednesday, July 1, 2020, Canadians celebrated Canada Day, and Saturday, July 4, is Independence Day – commonly referred to as the Fourth of July – in the United States. That means it's fireworks season in North America, though many – if not most – displays have been canceled in 2020 due to the pandemic.
So you might or might not see fireworks (a type of pyrotechnics) this year. Still, it's fun to recall the reds, oranges, yellows, greens, blues and purples exploding in the skies, creating "ohhhhs" and "ahhhhs."
And it's interesting to know what creates those brilliant colors.
The colors in fireworks come from a simple source: pure chemistry. They're created by the use of metal salts. These salts are different from table salt, and in chemistry 'salt' refers to any compound that contains metal and non-metal atoms. Some of these compounds produce intense colors when they are burned, which makes them ideal for fireworks. Others, like potassium nitrate, sulfur and charcoal are often used to help the fireworks burn. Nitrates, chlorates and perchlorates provide oxygen for the combustion of the fuel. Dextrin, often used as a starch, holds the mixture together. Some colors can be strengthened with the use of chlorine donors.
Read: It's July 4, 2020, but the fireworks shows won't go on
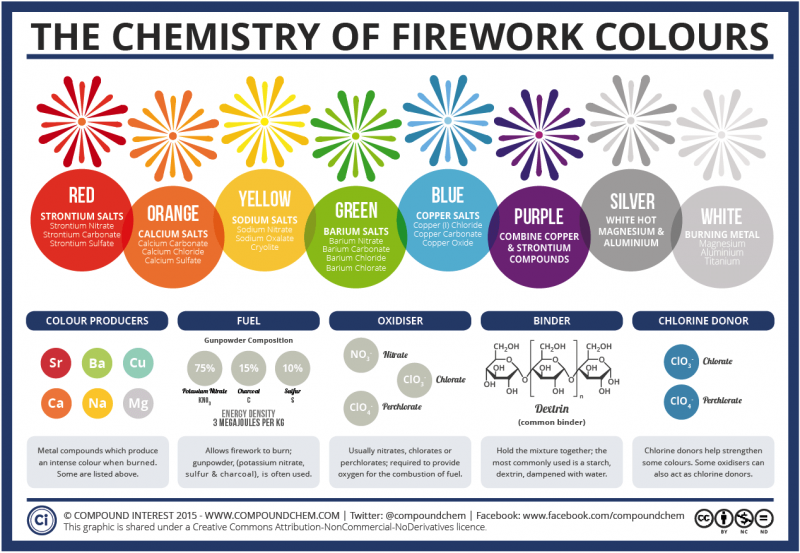
Metal salts commonly used in firework displays include: strontium carbonate (red fireworks), calcium chloride (orange fireworks), sodium nitrate (yellow fireworks), barium chloride (green fireworks) and copper chloride (blue fireworks). Purple fireworks are typically produced by use of a mixture of strontium (red) and copper (blue) compounds.
The metal salts are packed into small pea- to plum-sized pellets called "stars" or "pyrotechnic stars."
After a firework is ignited, a lift charge propels it into the sky. That's just explosive black powder in a confined space that, when lit, causes a fast increase of heat and gas that can send a firework as high as 1,000 feet (300 meters) into the air.
Meanwhile, a time-delay fuse burns slowly into the interior of the firework shell. After about 5 seconds, as the shell is soaring overhead, the fuse kindles a charge that reaches the core of the firework, explodes and ignites the stars that contain the metal salts.

Voila! A beautiful and colorful fireworks display.
By the way, the people who create fireworks are precise, expert craftsmen. Putting on a fireworks display is a complex process, and must be done safely in a controlled environment. If even one thing is off — too much black powder, stars that aren't aligned correctly or a trigger that fires too soon or too late — everything can go kaboom. Fireworks are explosives, after all, and working with them is best left to the professionals.
Bottom line: The red, orange, yellow, green, blue and purple colors exploding in the night sky during a fireworks festival are created by the use of metal salts. In 2020, many if not most fireworks displays have been canceled due to the pandemic.
Read more: The Chemistry of Fireworks Colors
Read more: The Chemistry of Fireworks
Enjoying EarthSky? Sign up for our free daily newsletter today!
culpinthipstrealm1983.blogspot.com
Source: https://earthsky.org/human-world/how-do-fireworks-get-their-vibrant-colors/
0 Response to "What Element Is Used to Create the Blue Color in Fireworks"
Post a Comment