what is a contraindication to an mmr booster for an adolescent female?
| A) | Centers for Illness Command and Prevention | ||
| B) | Informational Committee on Immunization Practices | ||
| C) | FDA Center for Biologics Evaluation and Research | ||
| D) | FDA Vaccines and Related Biological Products Informational Commission |
AN OVERVIEW OF IMMUNIZATION SCHEDULES
It is helpful to understand how vaccines are canonical and and so recommended as role of a schedule. The U.Southward. Food and Drug Administration's (FDA) Center for Biologics Evaluation and Enquiry (CBER) is responsible for regulating vaccines in the United States. Vaccine clinical development follows the same general pathway as drugs and other biologics. A sponsor who wishes to begin clinical trials with a vaccine must submit an investigational new drug application (IND) to the FDA. The IND describes the vaccine, its method of manufacture, and the types of quality control testing washed prior to administering the vaccine to humans. Besides included is information about the vaccine'due south safety and ability to elicit an immune response in animal testing. In addition, the IND contains the proposed clinical protocol.
two . According to the 2022 immunization schedule, what are the recommended vaccine doses for a salubrious, 2-month-old infant with no special risks or contraindications who is upwardly-to-appointment on vaccinations so far?
| A) | DTaP, Hib, IPV, and HepB if needed | ||
| B) | DTaP, Hib, PCV, IPV, and HepB if needed | ||
| C) | Rotavirus, DTaP, Hib, IPV, and HepB if needed | ||
| D) | Rotavirus, DTaP, Hib, PCV13, IPV, and HepB if needed |
AN OVERVIEW OF IMMUNIZATION SCHEDULES
RECOMMENDED IMMUNIZATION SCHEDULE FOR PERSONS 0 THROUGH 18 YEARS OF AGE, 2019
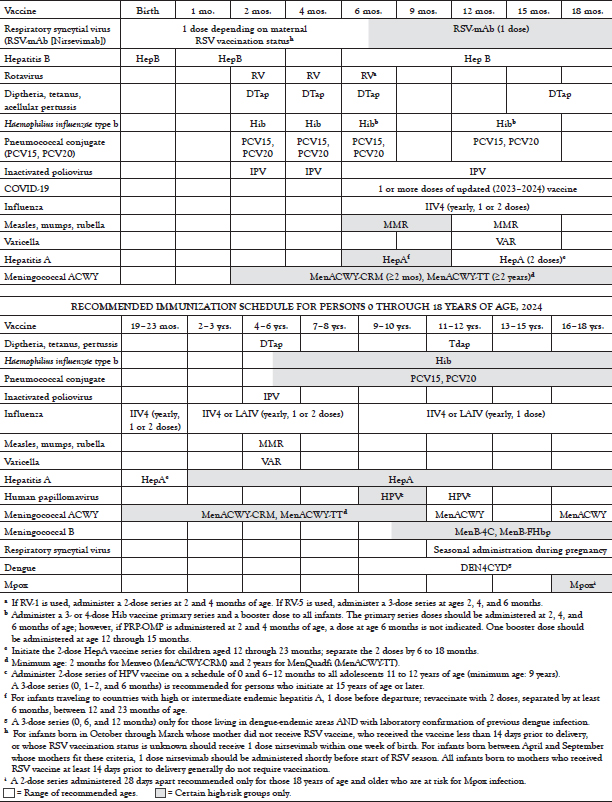
3 . Assuming no special risk groups or contraindications and bold that the ACIP recommendations are followed, what vaccines would a male patient, 50 years of age, be likely to receive?
| A) | Tdap or Td simply | ||
| B) | Tdap or Td, IIV, and zoster | ||
| C) | Tdap or Td, IIV, and PPSV23 | ||
| D) | Tdap or Td, IIV, zoster, and PPSV23 |
AN OVERVIEW OF IMMUNIZATION SCHEDULES
RECOMMENDED Adult IMMUNIZATION SCHEDULE Past VACCINE AND Historic period Group, 2022
| Vaccine | 19–23 years | 24–26 years | 27–49 years | fifty–64 years | 65 years and older | ||||
|---|---|---|---|---|---|---|---|---|---|
| Influenza (IIV4, RIV4, or LAIV4) | 1 dose (IIV4, RIV4, or LAIV4) annuallya | 1 dose (IIV4 or RIV4) annuallya | |||||||
| Tetanus, diphtheria, pertussis (Td or Tdap) | One dose of Tdap, so boost with Tdap or Td every x years | ||||||||
| One dose Tdap during each pregnancy; 1 dose for wound prophylaxisa | |||||||||
| Varicella | two doses (if born in 1980 or later)a | 2 dosesb | |||||||
| Human being papillomavirus | ii or 3 dosesa | ii or 3 dosesc | — | — | |||||
| Zoster (RZV) | ii doses (if immunocompromised)b | ii doses | |||||||
| Measles, mumps, rubella | 1 or 2 doses (if born 1957 or later)a | — | |||||||
| Pneumococcal 13-valent conjugate (PCV15, PCV20, PPSV23) | 1 dose PCV20 OR 1 dose PCV15 followed by PPSV23b | i dose PCV20 OR 1 dose PCV15 followed by PPSV23a | |||||||
| Hepatitis A | ii or 3 dosesb | ||||||||
| Hepatitis B | ii, iii, or 4 doses | 2, 3 or 4 dosesb | |||||||
| Meningococcal ACWY | 1 or 2 doses, then boosterb every 5 years | ||||||||
| Meningococcal B (MenB) | 2 or 3 dosesc | ii or 3 dosesb | |||||||
| Haemophilus influenzae type b (Hib) | 1 or 3 dosesb | ||||||||
| |||||||||
iv . The ACIP rationale for expanding the recommendation for flu vaccination to include all children from six months to eighteen years of historic period includes
| A) | adolescents typically have loftier rates of dr. visits. | ||
| B) | universal babyhood vaccination is expected to help increase coverage for at-risk groups. | ||
| C) | missed schoolhouse days due to influenza have been low simply proven to adversely affect children's grades. | ||
| D) | a large new clinical trial reinforced conviction in the safety and efficacy of flu vaccination in schoolhouse-age children. |
VACCINES AND RECOMMENDATIONS
The expansion of the recommended ages for the vaccination of children and adults confronting flu is ane of the most meaning changes to the schedule in recent years. Information technology requires an almanac visit to a healthcare provider, including among older children and young adults who typically take low rates of physician visits.
The ACIP considered multiple factors in making this recommendation. Start, according to accumulated testify, the influenza vaccine appears to be both safe and effective, with the benefits of vaccination outweighing the small adventure of adverse furnishings [21]. Widespread vaccination is also intended to lower the social and economic touch of influenza. The number of missed days of schoolhouse for children and missed days of piece of work for parents is substantial. Physician visits for the flu may lead to a prescription for antibiotics—treatment that is unnecessary and potentially dangerous.
The recommendation is also intended to simplify the decision to advise vaccination for children [21]. In previous years, vaccination was recommended for a number of groups with specific risk factors. These included older children with certain medical weather and children who were close contacts of people who should be immunized. Making vaccination routine for all children is expected to lead to a 50% increase in coverage for those children who have a specific risk-based or contact-based indication.
v . According to the ACIP recommendations, and considering good for you patients without special take chances factors or contraindications, who should receive the HPV vaccine?
| A) | Girls 11 to 12 years of historic period, plus developed women at loftier risk of contracting HPV | ||
| B) | Girls xv years of age or older, plus adult women at high take a chance of contracting HPV | ||
| C) | Girls younger than 18 years of historic period who are sexually active, plus adult women through age 26 who have not been vaccinated | ||
| D) | All individuals 11 to 26 years of age who accept not been vaccinated |
VACCINES AND RECOMMENDATIONS
Recommendation for Adolescents: HPV vaccine is recommended for girls and boys 11 to 12 years of age and for older adolescents who have not yet been vaccinated. Children nine to 10 years of age may too be vaccinated.
Recommendation for Adults: HPV vaccine is recommended for adults up to 26 years of age who have not completed the vaccine series. HPV vaccine is also recommended for those 27 to 45 years of historic period if desired or if a risk factor is present.
6 . The ACIP rationale for recommending HPV vaccination to preteens includes all of the following, EXCEPT:
| A) | HPV infection is particularly common in teenagers and young adults. | ||
| B) | Vaccination before the age of sexual debut is probable to offer the most benefit. | ||
| C) | Infection with HPV frequently occurs inside the first few years after sexual debut. | ||
| D) | Afterward an private has been infected with any type of HPV, the vaccine is no longer of benefit. |
VACCINES AND RECOMMENDATIONS
Epidemiologic data on HPV incidence and age of sexual debut suggest that the pre-teen years are an appropriate time to begin HPV protection [32]. Genital HPV is the most common sexually transmitted infection in the Usa, with 13 million new infections among people ≥15 years of age each year [95]. Teens and young adults are particularly at risk; about half of those infections occur in individuals 15 to 24 years of age [32]. One multisite, clinic-based report of sexually agile females found the highest prevalence of HPV in girls fourteen to 19 years of age. In another study, using a representative, population-based sample, HPV prevalence was 26.9% among sexually active women 18 to 25 years of historic period [33]. The prevalence of types 16 or 18 was seven.8%. Some other study, too intended to exist representative of the general population, found that the prevalence of HPV was 26.8% for women 14 to 59 years of age and nearly 45% among women 20 to 24 years of age [34]. In the overall written report population, the prevalence of type xvi was 1.5%, and type 18 was 0.8%.
An important consideration in protecting adolescents who are not even so sexually active is that HPV infection is mutual within the beginning few years after sexual debut [32]. In add-on, studies have shown high antibiotic titers with vaccination at age 11 to 12 years. The projected affect of vaccinating girls at 12 years of age is a 20% to 66% reduction in lifetime cervical cancer run a risk, depending on the effectiveness of the vaccine and the duration of protection. Vaccination could also lead to a 21% reduction in low-course abnormalities on Pap tests over the life of a accomplice of vaccinated females. A comparing of HPV prevalence data from the vaccine era (2009–2012) and the prevaccine era (2003–2006) establish that the prevalence of the HPV types included in the quadrivalent vaccine decreased past 64% (from 11.5% to four.three%) among girls fourteen to 19 years of historic period [17]. Because the modest uptake of this vaccine, the potential impact is significant.
The recommendation to vaccinate young adults takes into business relationship the fact that many will already be sexually active and may have been exposed to 1 or more types of HPV. Young adults who are non all the same sexually active can receive the full do good of vaccination. In add-on, it is likely that many individuals who are infected have not yet encountered each of the vaccine-covered types, and then they tin can receive at to the lowest degree partial benefit [35,36]. The recommendation to vaccinate adults to the age of 26 years reflects the rubber and efficacy testing on which the initial vaccines' approvals were based [30,31,37]. Apply in older individuals is also effective, and many patients will do good from vaccination at 27 to 45 years of age. Medical professionals tin can inform patients of the selection to receive the vaccine series or to complete the series, assistance assess the benefits and individual risk factors, and facilitate decision-making. As noted, the HPV vaccine remains significantly underutilized as of 2022.
7 . What modify was fabricated to the recommendations regarding vaccination against rotavirus in 2009?
| A) | Three rotavirus vaccines are at present bachelor. | ||
| B) | Ages for dosing were harmonized for the two available vaccines. | ||
| C) | The number of doses was standardized, with both vaccines now requiring 2 doses. | ||
| D) | The age to initiate rotavirus vaccination was expanded to include infants upwards to 1 year of historic period. |
VACCINES AND RECOMMENDATIONS
In 2009, the age parameters for vaccine administration were adapted to harmonize the schedules of the ii approved rotavirus vaccines [twoscore]. One is a pentavalent reassortant vaccine based on a bovine rotavirus, oftentimes abbreviated as RV5. The other is a live, attenuated human rotavirus vaccine, ofttimes abbreviated equally RV1. RV5 has a iii-dose schedule, while RV1 requires two doses [41]. The maximum ages for these vaccines are somewhat different, according to their prescribing information, but an ACIP workgroup has concluded that rubber and efficacy are unlikely to be affected if the same age limits are used for both [twoscore].
8 . Why is MCV included every bit a routine vaccination for healthy children?
| A) | Unlike MPSV, MCV covers all of the most common meningococcal serotypes. | ||
| B) | The loftier number of cases, well-nigh 45,000 in the United States each year, makes vaccination essential. | ||
| C) | Vaccinating children protects them confronting meningococcal affliction in middle age, when incidence becomes highest. | ||
| D) | In addition to the loftier case-fatality rate, each case of meningococcal illness requires substantial resources to place additional cases and forestall disease spread. |
VACCINES AND RECOMMENDATIONS
Historically, before widespread vaccination, there were almost 1,400 to ii,800 cases of meningococcal disease in the United States each yr [42]. Although not a common affliction, meningococcal disease has a rapid course and a high caste of mortality, with a case-fatality ratio of nearly ten% to 14%. Among survivors, eleven% to 19% will experience serious sequelae, such as neurologic deficit, deafness, or loss of a limb [43]. The caste of severity means that, in addition to putting the patient's life at risk, each case requires a substantial public health effort to place additional cases quickly and prevent the illness from spreading [44].
nine . The zoster vaccine is included on the adult immunization schedule. The recommendation for this vaccine includes
| A) | adults 50 years of historic period and older. | ||
| B) | adults 65 years of historic period and older. | ||
| C) | only adults with certain medical risk factors. | ||
| D) | but adults who have never had chickenpox. |
VACCINES AND RECOMMENDATIONS
Recommendation for Adults: RZV is recommended for individuals fifty years of age and older with no vaccination history and for individuals who previously received the ZVL vaccine. RZV is also recommended for individuals 19 years of age or older who are immunocompromised or who will be immunodeficient/immunosuppressed due to illness or therapy.
10 . Before vaccination was bachelor, what proportion of the population experienced herpes zoster at some point in their lives?
| A) | Nearly 1-10th | ||
| B) | About one-third | ||
| C) | About one-half | ||
| D) | Well-nigh 2-thirds |
VACCINES AND RECOMMENDATIONS
In that location are an estimated ane million cases of herpes zoster each year, and incidence increases with age [49]. Without vaccination, nearly 1-third of Americans will experience shingles at some point in their lives [49]. In addition to discomfort and inconvenience for the patient, in that location is likewise a risk of viral transmission leading to primary varicella in at-risk contacts. Postherpetic neuralgia (PHN) is an unfortunate but fairly common complication. A customs-based report in Minnesota looked at the incidence of PHN as divers by various durations of pain [50]. Xviii percent of patients experienced PHN-type pain for at least 30 days, 13% for at least sixty days, and 10% for at least 90 days [l]. The ACIP added the zoster vaccine to the developed immunization schedule to take reward of the opportunity to subtract both the brunt of disease and the risk of complications. In 2018, the recombinant zoster vaccine (RZV) was added as the preferred vaccine, and in 2020, the ZVL vaccine was discontinued [nineteen]. RZV has better proven efficacy in preventing herpes zoster compared with ZVL, and breakthrough cases are associated with less severe herpes zoster-related pain and less interference on activities of daily living [56].
11 . If a patient has a astringent (anaphylactic) latex allergy, how would this affect the vaccinations he or she could receive?
| A) | No vaccinations should be given. | ||
| B) | Some vaccines would be contraindicated. | ||
| C) | All vaccines tin exist used, but fifteen minutes of observation is recommended. | ||
| D) | There would be no modify, because latex is non used in manufacturing vaccines. |
VACCINE CONTRAINDICATIONS
HYPERSENSITIVIES AND VACCINE RECOMMENDATIONS
| Hypersensitivity | Vaccine | CDC Recommendation | |||||
|---|---|---|---|---|---|---|---|
| Yeast |
| Do non utilise | |||||
| Eggs | Influenza (LAIV, RIV, IIV) | Utilise with cautiona | |||||
| Latex | Rotavirus (RV1), MenB | Cheque packaging to see if latex is used and for guidance | |||||
| Gelatin |
| Utilize extreme caution if administering | |||||
| Neomycin |
| Do non use | |||||
| Streptomycin | IPV | Do non use | |||||
| Polymyxin B |
| Do not utilise | |||||
| Thimerosal | Some brands/formulations, including certain DTaP, influenza (IIV), Td, DT | Check parcel insert | |||||
| aProtocols accept been devised for administering IIV to patients with egg allergies. | |||||||
12 . A male parent brings his 5-year-old son, Patient S, in for a checkup i morning in November. He states that Patient Due south has had "the sniffles" for the past ii days and that he has been "running a bit of a fever." On test, Patient South appears well except for nasal congestion. His temperature is 99.0°F. Patient Due south'southward medical history is unremarkable, he has no known allergies, and he tolerated his previous vaccinations well. He was up-to-date on all recommended vaccinations through 2 years of age, but has non received any vaccinations since then. At today's visit, which of the following vaccines should probably be deferred?
| A) | IPV | ||
| B) | DTaP | ||
| C) | MMR | ||
| D) | LAIV |
VACCINE CONTRAINDICATIONS
The ACIP recommends that LAIV not be used in patients with asthma or other conditions predisposing to flu complications [12,21]. In nigh cases, IIV or another type tin can be used instead. LAIV should also exist avoided in children and adolescents who are receiving aspirin or salicylate therapy. Acute respiratory illness with nasal congestion, which could interfere with delivery of the vaccine, is a reason to consider delaying the use of this vaccine until the congestion has decreased. Children younger than five years of age who have recent or recurrent wheezing should not receive LAIV [12,21].
13 . A mother brings her young daughter to a new pediatrician for the get-go time. She is irresolute doctors because her previous pediatrician refuses to run into patients whose parents turn down to have them vaccinated. She explains, "I know that MMR vaccine can cause autism, and I don't want that to happen to my kid." What can you tell her?
| A) | Large observational studies have failed to find a link between MMR and autism. | ||
| B) | Experts practice non believe that MMR causes autism, but this issue has non been studied. | ||
| C) | An older type of MMR was a crusade of autism, just this specific vaccine is no longer used. | ||
| D) | Good prove links MMR and autism, but the benefits of vaccination are considered to outweigh the risks. |
VACCINE Rubber
Although measles was considered effectively eliminated in the United States in 2000, resurgence in the disease and regional outbreaks have resulted from suboptimal vaccination rates. In 2014, at that place were 667 cases of measles in the United States, more than ten times the number of cases in 2000; another even larger spike occurred in 2019 (ane,282 cases in 31 states) [six]. A big outbreak in 2014–2015 was linked to unvaccinated children visiting Disneyland, the source patient probably existence infected overseas (probable the Philippines) [6]. The decrease in vaccine coverage is in part attributed to the false belief that the MMR vaccine may cause autism. Based on multiple studies, experts generally concur that there is no evidence for a link between the MMR vaccine and autism, and information technology is important that clinicians address these misconceptions with patients. In 2004, the Institute of Medicine (IOM) reported that "the body of epidemiological evidence favors rejection of a causal relationship between the MMR vaccine and autism" [83]. The American University of Pediatrics has also concluded that the bear witness does not support such a connection. In add-on, autism is not idea to be immune-mediated, and there is no articulate mechanism by which MMR would cause this disorder [84].
Research on the topic includes a Canadian study involving 27,749 children born between 1987 and 1998 [85]. This written report found no association between rates of pervasive developmental disorder and either one or 2 doses of the MMR vaccine. In a 2015 retrospective accomplice study of 95,727 children, MMR vaccine receipt was not found to predict autism diagnosis, even amongst children with older siblings with an autism spectrum disorder [78]. A study of 657,461 children built-in in Denmark between 1999 and 2010 establish no increased run a risk of autism in those who received the MMR vaccine, including in special subgroups (east.g., autism hazard factors, other childhood vaccinations) [115].
fourteen . Some parents take concerns almost the presence of thimerosal in childhood vaccines. Which of the following is correct?
| A) | Experts believe that thimerosal does not crusade autism, only this has not been studied. | ||
| B) | Thimerosal remains a component of virtually childhood vaccines, only observational studies take not institute a connection with autism. | ||
| C) | Vaccines recommended for children 6 years of historic period and younger now either contain no thimerosal or contain but trace amounts, because thimerosal was shown to cause autism. | ||
| D) | Vaccines recommended for children 6 years of historic period and younger now either contain no thimerosal or incorporate only trace amounts, although observational studies have non found a connection betwixt thimerosal and autism. |
VACCINE Safety
Some of the concerns well-nigh autism involve the use of thimerosal, a mercury-containing preservative. The IOM has ended that, every bit with concerns near MMR, the testify favors rejecting the idea of a causal relationship between thimerosal-containing vaccines and autism [83]. In addition, the same study that looked at MMR and autism in a large cohort of Canadian children also looked for whatever relationship betwixt ethylmercury exposure and autism and failed to notice a connection [85]. Exposure levels were comparable to levels in the U.s.a. during the 1990s. Another study, which examined the incidence of autism in California children before and later thimerosal was removed from childhood vaccines, establish no decrease in autism following the change [88].
Most vaccines for children 6 years of age or younger that had independent thimerosal either no longer comprise this preservative or incorporate only trace amounts—small enough that the FDA considers them "preservative gratuitous" [89]. IIV vaccines are now largely in this category, as "preservative-free" preparations of IIV are widely available. For the 2021–2022 season, 87% of IIV vaccines are thimerosal-gratis or thimerosal-reduced formulations [118].
xv . A woman, 70 years of age, who is in generally skilful wellness, comes in to discuss some knee pain she has been having. While she is in your office, you take advantage of the opportunity to offer vaccination against seasonal influenza. She tells you lot that ane of her friends is recovering from Guillain-Barré syndrome (GBS), and she recalls hearing something about the flu shot and GBS. What can you tell her?
| A) | In that location is a proven risk with some of the current influenza vaccines, but non all. | ||
| B) | The rumor that incidence of GBS increased with the 1976 swine flu vaccine is untrue. | ||
| C) | There is a proven risk with the current influenza vaccines, just it is pocket-sized, about 1 case per ane million people. | ||
| D) | At that place is a theoretical risk with the electric current influenza vaccines, but fifty-fifty if there is a gamble information technology would probably exist pocket-size, about one case per 1 million people. |
VACCINE Safe
GBS was associated with a swine influenza vaccine in 1976, with an estimated 1 case per 100,000 people vaccinated [21]. Some observational studies since and then have found a small increase in GBS cases associated with flu vaccination, while others have found no link. Whether there is an clan between current influenza vaccines and GBS is not known. According to the CDC, based on studies in prior seasons, if an association does exist the chance would probable be low (i.e., one example per 1 million people vaccinated). The IOM conducted a thorough scientific review of this issue in 2003 and ended that people who received the 1976 swine influenza vaccine had an increased adventure for developing GBS. Scientists have multiple theories regarding why this increased risk may have occurred, but the exact reason for this clan remains unknown [93].
16 . As of 2022, what is known about HPV and problems post-obit vaccination?
| A) | The bulk of events reported to VAERS take been considered not-serious. | ||
| B) | Postmarketing reports rule out whatsoever connection between vaccination and syncope. | ||
| C) | The simply events reported to VAERS accept been non-serious, such as fainting, swelling at the injection site, headache, nausea, or fever. | ||
| D) | All of the above |
VACCINE Safe
Clinical trials and the post-licensure monitoring information of three HPV vaccines (two discontinued and one in current use) show that they are safe [107]. Since the licensure of the HPV vaccines, both the CDC and the FDA take monitored HPV vaccine safety through VAERS, VSD, and CISA systems. It should be noted that nearly of the available data is from the quadrivalent Gardasil conception, which is no longer available in the United States. A 2009 CDC/FDA written report found that the most common adverse events reported to VAERS post-obit vaccination with Gardasil were fainting, swelling at the injection site, headache, and nausea. Vii percent were considered serious. Nonetheless, no common pattern for serious events has emerged, making it hard to form theories nearly causality. GBS was reported but did not announced to occur at a charge per unit above groundwork levels. Claret clots were reported in a pocket-size number of patients, near of whom had pre-existing risk factors (e.g., smoking, obesity, use of oral contraceptives). Over the commencement three years of its use, more than than 28 million doses of Gardasil ix were administered, and seven,244 adverse events were reported to VAERS, of which 3% (217 events) were classified as serious [107].
17 . Research regarding parents' concerns about vaccination suggests that
| A) | it is unusual for parents to have questions or concerns about vaccines. | ||
| B) | the majority of parents have some level of uncertainty well-nigh vaccinating their children. | ||
| C) | information from healthcare providers is unlikely to influence decisions about vaccination. | ||
| D) | data from healthcare providers can have an important touch on parents' decisions to vaccinate. |
OVERCOMING BARRIERS FOR CHILDREN AND ADOLESCENTS
Healthcare providers can have an influence when parents are concerned or dislocated about vaccines. For example, in one survey, 28% of parents had some level of uncertainly nearly vaccines [97]. For those who ultimately decided to allow timely vaccination, assurances or information provided by a healthcare provider were of import reasons for the decision.
xviii . In addition to children who are enrolled in Medicaid, children who are eligible for costless vaccines nether the Vaccines for Children program include children who
| A) | are underinsured. | ||
| B) | have no health insurance coverage. | ||
| C) | are American Indian or Alaska Native. | ||
| D) | All of the above |
OVERCOMING BARRIERS FOR CHILDREN AND ADOLESCENTS
The Vaccines for Children (VFC) plan is designed to help overcome cost as a barrier to childhood vaccination. All of the ACIP-recommended vaccines are available for children enrolled in Medicaid, with VFC covering children through 18 years of age [101]. Children who have no health insurance coverage, children who are underinsured, and children who are American Indian or Alaska Native are besides eligible for vaccines through VFC.
19 . You lot have found that there is room for improvement in pediatric vaccination rates. Ane of the nurses suggests sending letters to remind both developed patients and the parents of pediatric patients when vaccinations are needed. Nonetheless, your part managing director reminds you that the budget is tight this year. Sending letters would be an extra expense. Based on evidence and electric current recommendations, what should you practice?
| A) | Either ship the letters or found a system of reminder phone calls. | ||
| B) | Send messages but for pediatric patients, because reminders work for children but non adults. | ||
| C) | Do not use messages or phone calls, because reminder systems for patients do non work. | ||
| D) | Found a system of reminder phone calls instead of letters, because calls take been proven to have greater effect. |
OVERCOMING BARRIERS FOR CHILDREN AND ADOLESCENTS
Reminding parents to bring their children in for vaccinations is a proven manner to increase coverage and is recommended in standards adult past the National Vaccine Advisory Committee and supported past other organizations [102,103]. Reminders need non take upwardly extensive staff fourth dimension. Mailed reminders have been shown to increment child vaccination rates and so have telephone calls, which may be computer-generated to salvage piece of work by the office staff [104,105,106]. Outreach should exist more intensive for families at high adventure of missing appointments [102].
twenty . Your group practice recently conducted a chart audit and discovered many "missed opportunities" for adult vaccination. Yous would like to institute a reminder system for yourself and your colleagues, but the others inquire if in that location is any evidence it will work. Based on the testify, what can you tell them?
| A) | A review of studies was inconclusive, simply a reminder arrangement volition practice no impairment and might assist. | ||
| B) | The office should simply use an electronic medical records system, because placing reminders in paper charts has been proven not to work. | ||
| C) | A review of studies plant that physician reminder systems, such equally chart notations, stickers, and patient lists, tin ameliorate vaccination coverage. | ||
| D) | Reminder systems for patients work, so even though reminder systems for physicians have not been studied, they can as well be expected to increase vaccination rates. |
OVERCOMING BARRIERS FOR ADULTS
There is evidence that when physicians recommend preventive services, patients are interested in receiving them. For example, 95.1% of patients in a national survey stated that they would accept the herpes zoster vaccination if their physician recommended it [111]. Standards provided by the National Vaccine Advisory Committee, in cooperation with more than sixty organizations, offer evidence-based methods to help reduce missed opportunities for adults [110]. Providers should assess the vaccination status of all new patients and review vaccination condition annually. Pneumococcal vaccination status should be reviewed when patients nowadays for influenza vaccination.
Standing orders for vaccination should be used, based on show that they improve developed vaccination coverage in many unlike settings [110]. Reminder systems for staff tin likewise meliorate vaccination rates. In 1 review of studies, use of doctor reminder systems, such as nautical chart notations, stickers, and patient lists, improved coverage by a median of 22% [112]. Assessing a practice'south success at vaccinating patients who are eligible and reporting the results to staff can likewise assistance to better coverage [110].
culpinthipstrealm1983.blogspot.com
Source: https://www.netce.com/studypoints.php?courseid=1754&printable=yes&page=printquestions
0 Response to "what is a contraindication to an mmr booster for an adolescent female?"
Post a Comment